At 850 k the equilibrium constant for the reaction – At 850 K, the equilibrium constant for a reaction plays a crucial role in determining the extent to which reactants convert into products. This article delves into the concept of equilibrium constant, its dependence on temperature, and its significance in predicting reaction direction and equilibrium position.
The equilibrium constant, denoted by K, is a measure of the relative concentrations of reactants and products at equilibrium. It provides valuable insights into the spontaneity and feasibility of a reaction under specified conditions.
Thermodynamic Parameters: At 850 K The Equilibrium Constant For The Reaction

The equilibrium constant, denoted by K, is a measure of the extent to which a reaction proceeds. It is a function of temperature and is related to the Gibbs free energy change (Δ G°) of the reaction by the equation:
ΔG° =
RTln K
where Ris the gas constant (8.314 J/mol·K) and Tis the temperature in Kelvin.
This equation shows that the equilibrium constant decreases as the temperature increases. This is because the Gibbs free energy change of a reaction is more negative at lower temperatures, which means that the reaction is more favorable at lower temperatures.
Reaction Quotient, At 850 k the equilibrium constant for the reaction
The reaction quotient, denoted by Q, is a measure of the relative amounts of reactants and products in a reaction at a given moment in time. It is calculated using the same expression as the equilibrium constant, but using the concentrations of the reactants and products at the current time rather than the equilibrium concentrations.
The reaction quotient can be used to predict the direction of a reaction. If Q < K, then the reaction will proceed in the forward direction to reach equilibrium. If Q> K, then the reaction will proceed in the reverse direction to reach equilibrium.
If Q= K, then the reaction is at equilibrium.
Factors Affecting Equilibrium
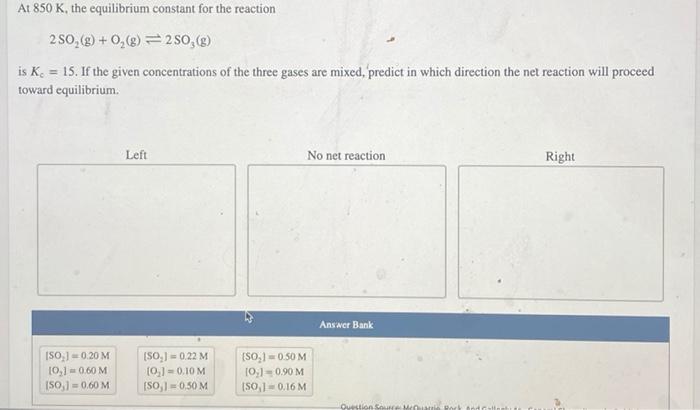
The equilibrium position of a reaction can be shifted by changing the following factors:
- Concentration of reactants and products
- Temperature
- Pressure (for gas-phase reactions)
- Addition of a catalyst
Changing the concentration of reactants or products will shift the equilibrium position in the direction that consumes the added substance. Increasing the temperature will shift the equilibrium position in the direction of the endothermic reaction. Increasing the pressure will shift the equilibrium position in the direction of the reaction that produces fewer moles of gas.
Adding a catalyst will speed up the reaction but will not affect the equilibrium position.
Applications of Equilibrium Constants
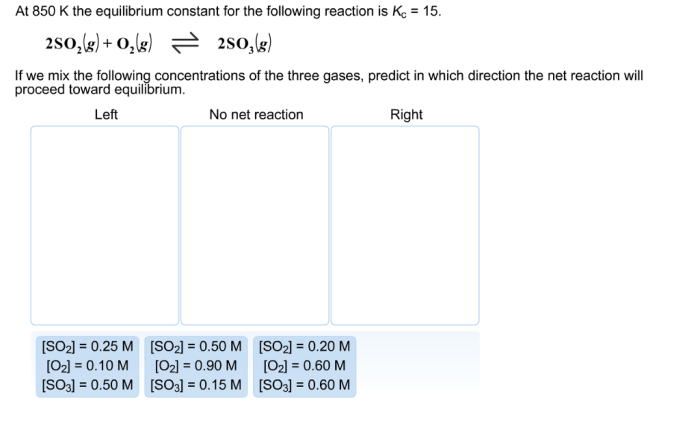
Equilibrium constants have a wide range of applications in chemistry, biochemistry, and environmental science. Some examples include:
- Calculating the pH of a solution
- Predicting the solubility of a solid in a solvent
- Designing chemical processes
- Understanding the behavior of biological systems
- Assessing the impact of environmental pollutants
Equilibrium constants are essential for understanding the behavior of chemical reactions and for solving a wide range of problems in chemistry and related fields.
Question Bank
What is the relationship between equilibrium constant and temperature?
The equilibrium constant is dependent on temperature. The van’t Hoff equation describes this relationship, showing that the equilibrium constant increases with increasing temperature for exothermic reactions and decreases with increasing temperature for endothermic reactions.
How is reaction quotient used to predict the direction of a reaction?
The reaction quotient, Q, compares the actual concentrations of reactants and products at any given time. If Q < K, the reaction proceeds in the forward direction to reach equilibrium. If Q > K, the reaction proceeds in the reverse direction to reach equilibrium.
What factors can affect the equilibrium position of a reaction?
Several factors can shift the equilibrium position, including changes in temperature, pressure, concentration, and the addition of a catalyst.